The spin magnetic moment per formula unit of ZnyFe3−yO4 for y = 0 and 1. | Download Scientific Diagram

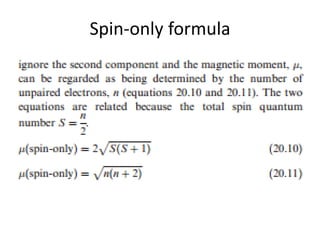
The magnetic moment (in BM) of Zn 2+ ion according to spin only formula is (a) Zero (b) 1.73 (c) 2.84 (d) 3.87 - Sahay Sir

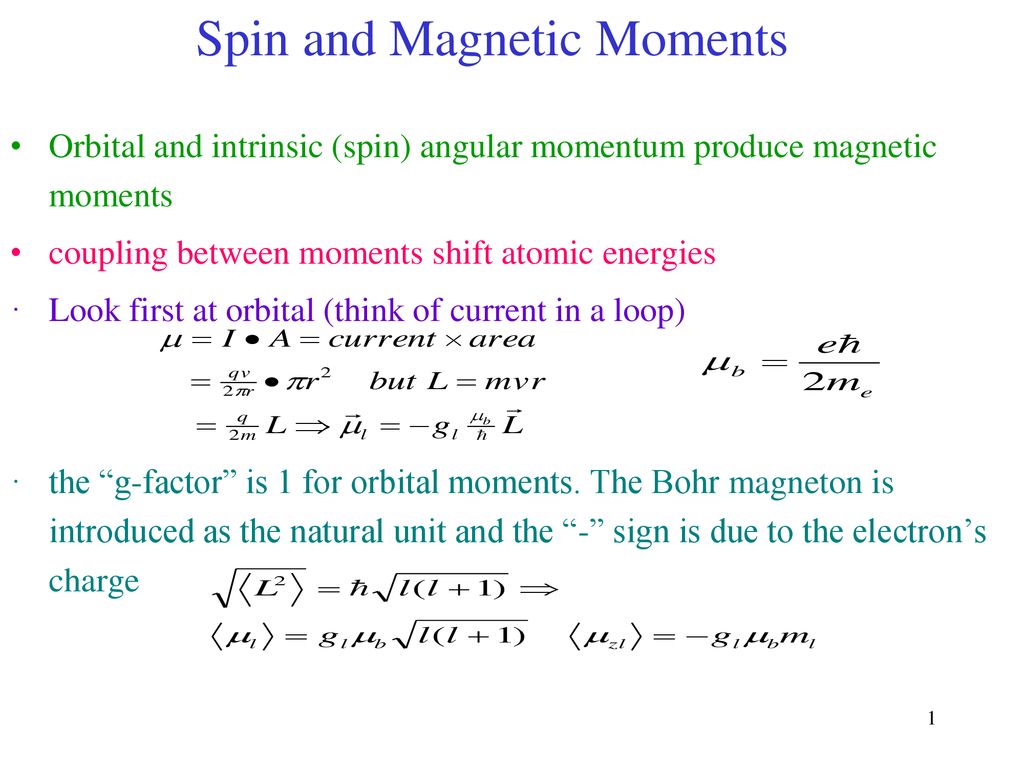
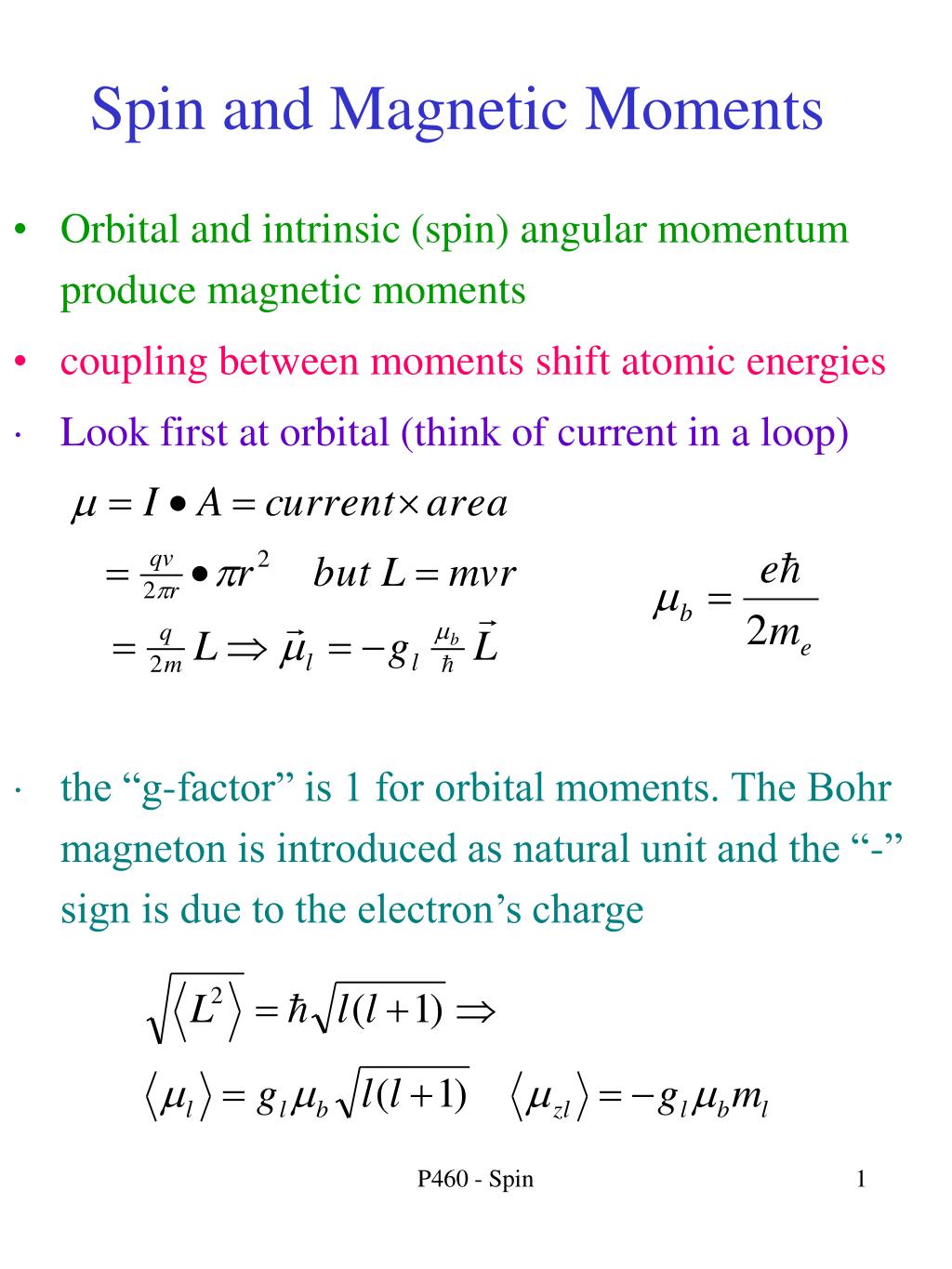
The correct electronic configuration and spin-only magnetic moment (BM) of Gd^3+ (Z = 64), respectively, are:

The highest value of the calculated spin-only magnetic moment (in BM) among all the transition - YouTube
![The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube](https://i.ytimg.com/vi/E3pLlLoxf48/maxresdefault.jpg)
The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM` On the basis of `VBT` Pr... - YouTube

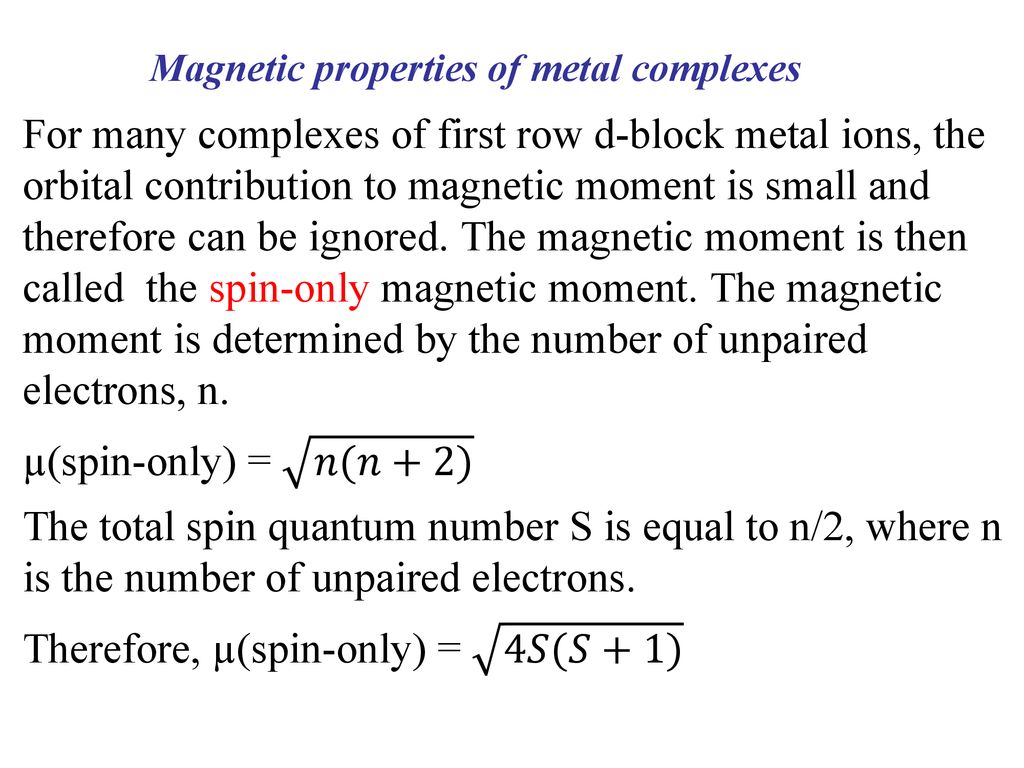
Question 8. Calculate the 'spin only' magnetic moment of M^{2+}(aq) ion(Z=27)Spin only magnetic moment depends upon the number of unpairedelectrons asmu =sqrt {n(n+2)}So first find the number of unpaired electrons from its
The calculated spin only magnetic moment of Cr^2+ ion is : (1) 2.84 BM (2) 3.87 BM (3) 4.90 BM (4) 5.92 BM - Sarthaks eConnect | Largest Online Education Community
The correct order of the spin-only magnetic moments of the following complexes is : - Sarthaks eConnect | Largest Online Education Community
![Ce is the first element of lanthanide series. What will be the 'spin only' magnetic moment of Ce^(3+) ? [Assume mu = sqrt(n(n+1)) B.M.] Ce is the first element of lanthanide series. What will be the 'spin only' magnetic moment of Ce^(3+) ? [Assume mu = sqrt(n(n+1)) B.M.]](https://d10lpgp6xz60nq.cloudfront.net/ss/web/534894.jpg)



![The magnetic moment (spin only) of [NiCl4]2– is - askIITians The magnetic moment (spin only) of [NiCl4]2– is - askIITians](https://files.askiitians.com/cdn1/cms-content/common/www.askiitians.comonlinetestforumsimages204-1316_sataug1615-54-35.jpg.jpg)